What Is Ozone? Unveiling The Science & Impact
Is the air we breathe friend or foe? Ozone, a seemingly simple molecule composed of three oxygen atoms, presents a fascinating duality: it can be both a life-saving shield and a dangerous pollutant, depending on where it resides.
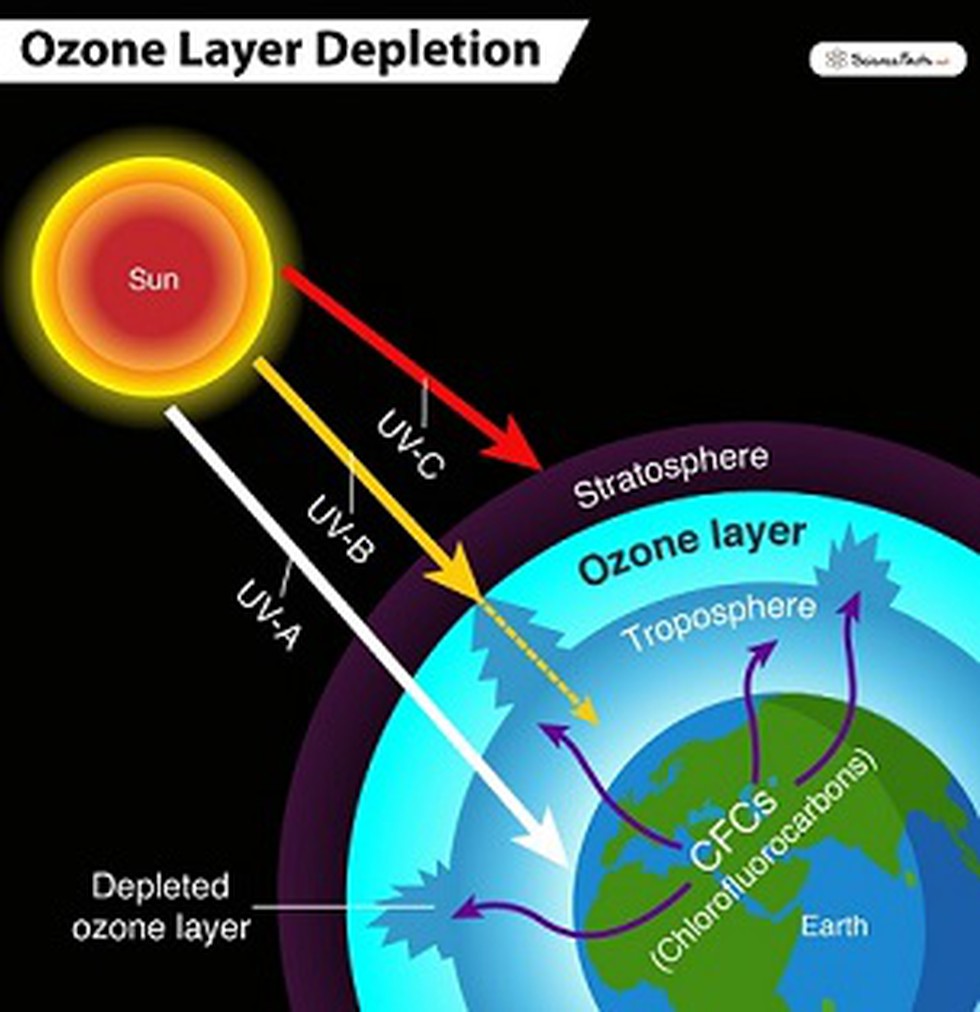
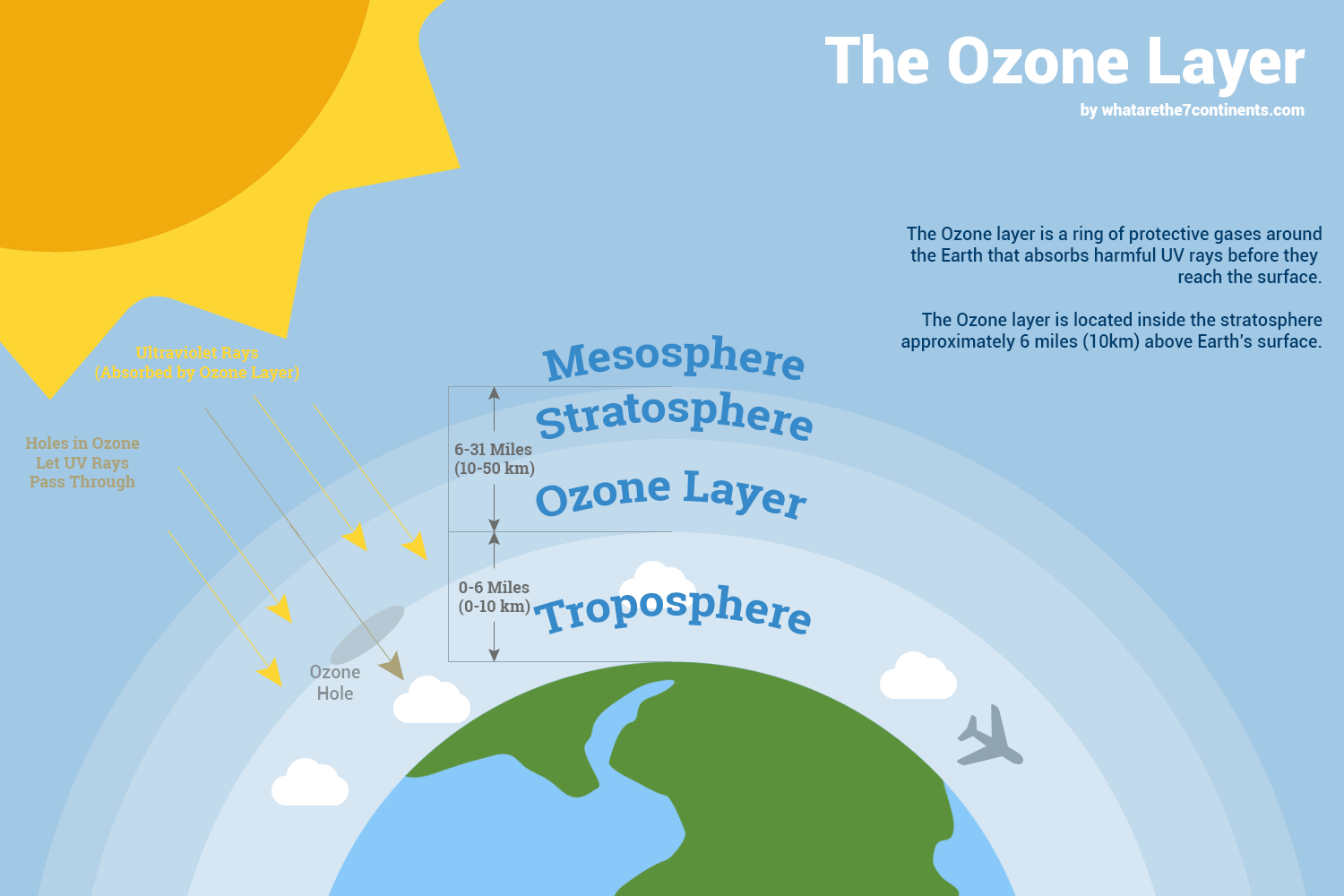
Ozone, a pale blue gas with a characteristic pungent odor, exists in two distinct realms: the stratosphere and the troposphere. In the upper atmosphere, specifically the stratosphere, the ozone layer acts as a crucial barrier, absorbing the sun's harmful ultraviolet (UV) radiation and shielding life on Earth. Without this protective layer, the intense UV rays would sterilize the planet's surface. This stratospheric ozone is our ally, a vital component for life as we know it.
However, at ground level, in the troposphere, ozone transforms into a serious air pollutant. Here, it is a byproduct of human activities, forming from the emissions of volatile organic compounds (VOCs) and nitrogen oxides (NOx) released by vehicles, industrial processes, and other sources. This ground-level ozone is a primary component of smog, posing significant health risks, particularly to those with respiratory problems. It can damage lung tissue, exacerbate existing conditions, and even contribute to premature death. Furthermore, it can harm plants, damage materials like rubber, and contribute to environmental degradation.
The very nature of ozone, its ability to readily react with other substances, contributes to its dual character. In the stratosphere, this reactivity allows it to absorb UV radiation, breaking down and reforming, a continuous cycle that filters the sun's rays. But at ground level, this same reactivity leads to the damage mentioned above.
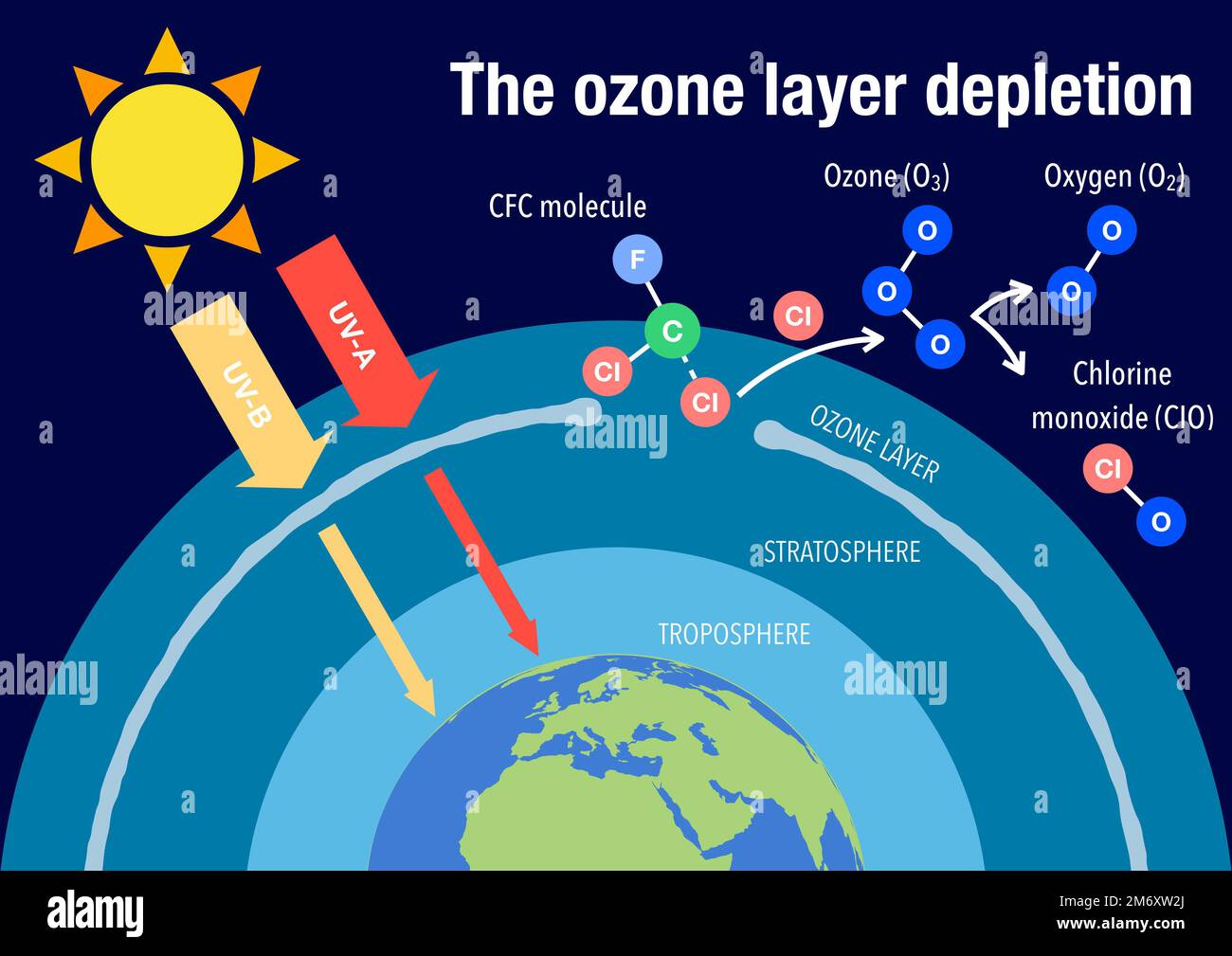
The formation of ozone is a complex process. In the stratosphere, it's primarily created through a natural process called photolysis, where UV radiation breaks down oxygen molecules (O2), and the individual oxygen atoms then combine with other oxygen molecules to form ozone (O3). In the troposphere, ozone formation is driven by sunlight and the presence of precursor pollutants, such as VOCs and NOx. These pollutants react with sunlight, triggering a cascade of chemical reactions that lead to the creation of ozone.
The ozone layer, a region of the stratosphere located roughly 15 to 35 kilometers (9 to 22 miles) above the Earth's surface, is not a solid, uniform entity. It's a region of relatively high concentrations of ozone molecules. The ozone layer is a dynamic system, constantly being created and destroyed. Ozone is also very rare in our atmosphere, averaging about three molecules of ozone for every 10 million air molecules.
The impact of human activities on the ozone layer and ground-level ozone is significant. The release of certain chemicals, such as chlorofluorocarbons (CFCs) and other ozone-depleting substances, has thinned the stratospheric ozone layer, creating what are often referred to as "ozone holes," particularly over the polar regions. This thinning allows more UV radiation to reach the Earth's surface. These pollutants are now heavily regulated, and in a report released in early 2023, scientists noted that Earth's atmosphere is recovering.
Ground-level ozone is directly influenced by human activities through the emission of precursor pollutants. Reducing these emissions through the use of cleaner technologies, stricter regulations, and sustainable practices is crucial to improving air quality and protecting public health. The levels of ozone are being monitored by various organizations, with the implementation of interactive air quality maps to inform the public.
Ozone also has some practical applications. It is used as a powerful oxidizing agent for water purification, a disinfectant, and a bleach. However, these uses must be carefully controlled to avoid environmental and health risks.
In addition to these scientific perspectives, ozone also touches upon other areas, such as the sports industry, with companies providing services in this space. Ozone billiards, for example, has more than 20 years of experience in providing quality products at competitive prices. There are companies that focus on specific niche markets, for instance, ozone gymnastics apparel.
The dynamic nature of the atmosphere, the interplay of natural processes, and the impact of human activity all contribute to the complex story of ozone, a substance that exemplifies the interconnectedness of our world. Understanding this intricate molecule is paramount to safeguarding both our health and the environment.
| Aspect | Details |
|---|---|
| Chemical Formula | O3 (Triatomic Oxygen) |
| Physical State | Colorless gas (at low concentrations), Pale blue (at high concentrations) |
| Odor | Pungent, sharp |
| Formation in Stratosphere | Photolysis of O2 by UV radiation, followed by O + O2 -> O3 |
| Formation in Troposphere | Reactions between NOx and VOCs in the presence of sunlight |
| Location | Stratosphere (Ozone Layer), Troposphere (Ground Level) |
| Function in Stratosphere | Absorbs harmful UV radiation from the sun |
| Health Effects (Troposphere) | Respiratory problems, lung damage, eye irritation |
| Environmental Effects (Troposphere) | Damage to plants, rubber, and other materials |
| Human Impact | Release of ozone-depleting substances (CFCs), Emissions of NOx and VOCs |
| Regulation | Air quality standards, international agreements (e.g., Montreal Protocol) |
| Uses | Water purification, disinfection, bleaching |
| Status of Ozone Layer | Recovering due to reduced emissions of ozone-depleting substances. |
For more information, you can visit: EPA Ozone Information